Prostatype Gene Test
Test Introduction
Prostate cancer is one of the most common forms of cancer and approximately 1.3 million men are diagnosed annually. Prostatype Genomics AB specializes in the development, manufacturing, and marketing of the prognostic genetic test Prostatype®. Prostatype® is a genetic test that provides a comprehensive assessment of the aggressiveness of prostate cancer.
The Prostatype prognostic gene test helps doctors and patients make correct treatment decisions for individuals with prostate cancer. By minimizing over- and under-treatment, the quality of life for patients can be improved and resources can be saved within healthcare.
Using prostate tissue from the biopsy obtained at the time of diagnosis (i.e. no new sampling of the prostate is needed), the Prostatype® test system combines gene expression information from three stem genes with currently used clinical parameters (PSA, Gleason Score, and Tumor Stage) and calculates the so-called P-score, which shows the risk of dying from prostate cancer.
Test Description
Prostatype gene test uses a tissue sample (biopsy) that has already been taken in connection with the diagnosis. The test analyzes three specific genes – IGFBP3, F3 and VGLL3 – that are strongly linked to prostate cancer. The gene expressions are quantified and the results are combined with other important clinical data.
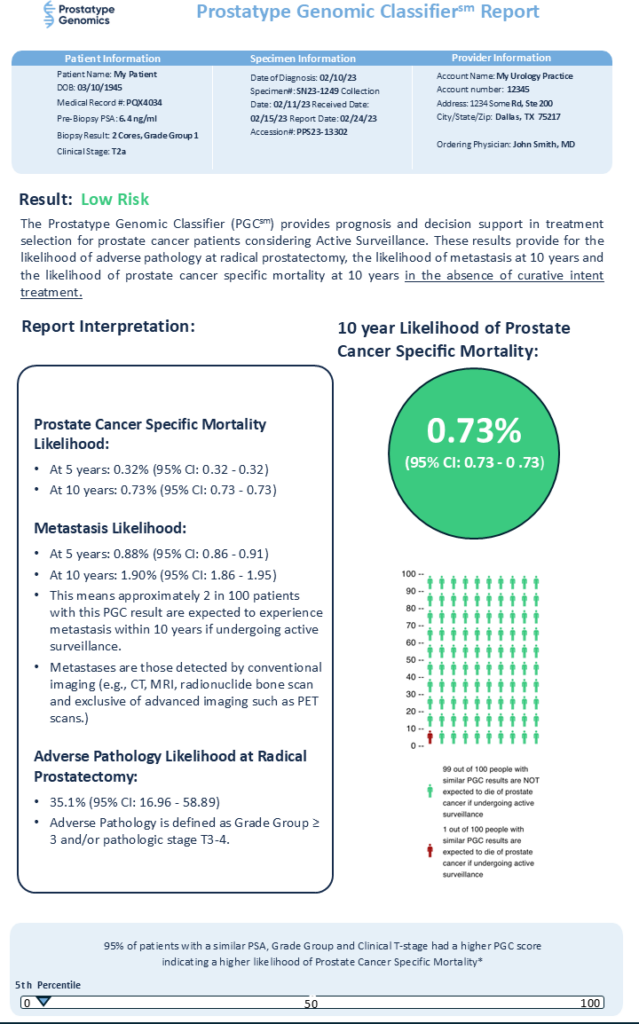
The test results from the genetic analyses are combined with PSA, Gleason score, and tumor stage to calculate a P-Score. The P-Score measures how aggressive or non-aggressive the cancer is. This combination of genetic and clinical data provides a comprehensive picture of the cancer’s nature and helps guide treatment decisions.
Calculation of P-Score
- Gene Analysis: The gene expressions of IGFBP3, F3 and VGLL3 are quantified from tumor biopsies.
- Clinical Parameters: PSA levels, Gleason score and tumor stage are used to complement the genetic data.
- Statistical Model: The Fine-Gray model, which handles multiple competing risks, is used to calculate the P-Score. This system weights each parameter using a carefully developed, verified, and validated algorithm and provides an accurate prediction of prostate cancer-specific mortality.
Risk categories
P-Scores are grouped into different risk categories (low, medium, high). Low risk indicates less aggressive cancer while high risk signals a more aggressive form of the disease
Test Ordering
The test may be ordered through the Prostatype Website. This test was developed, and its analytical performance characteristics have been determined by Prostatype Genomics. The test is performed in the Prostatype Genomics Clinical Laboratory located in the ResearchDx Incubator Facility. It has not been cleared or approved by FDA. This assay has been validated pursuant to the CLIA regulations and is used for clinical purposes.
Sample Collection and Shipping Instructions
For Tissues analysis, FFPE Tissue Blocks are the preferred sample type versus slides. If sending unstained slides, please send a minimum of 10-15 slides, freshly cut at 4-6 microns. Archival cut slides are acceptable if less than 30 days old.