FibroSIGHT: MASH
Test Introduction
FibroSIGHT, a Laboratory-Developed Test (LDT), developed for reliable and consistent MASH fibrosis assessment. FibroSIGHT exploits a unique stain-free imaging methodology finely tuned to the sensitive detection of collagen fibers – an excess of collagen fibers is the underlying cause of fibrosis – in liver biopsy samples. FibroSIGHT overcomes the inconsistencies associated with traditional staining methods and can integrate seamlessly into existing histopathology workflows. FibroSIGHT’s imaging readouts have the ability to empower clinicians to more objectively evaluate a patient’s condition, help inform treatment plans at diagnosis and to assist in evaluating response to treatments – crucially addressing the central role of managing liver fibrosis in MASH patients.
Test Description
FibroSIGHT leverages HistoIndex’s advanced proprietary stain-free imaging and digital pathology platform developed on Second Harmonic Generation (SHG) and Two-Photon Excitation Fluorescence (TPEF). SHG imaging detects fibrillar collagen in biological tissues with consistency, high-resolution and excellent signal-to-noise ratio, while TPEF allows visualization of pertinent cell structures – all without the use of any exogenous dyes. This overcomes the limitations and variability of traditional staining methods.
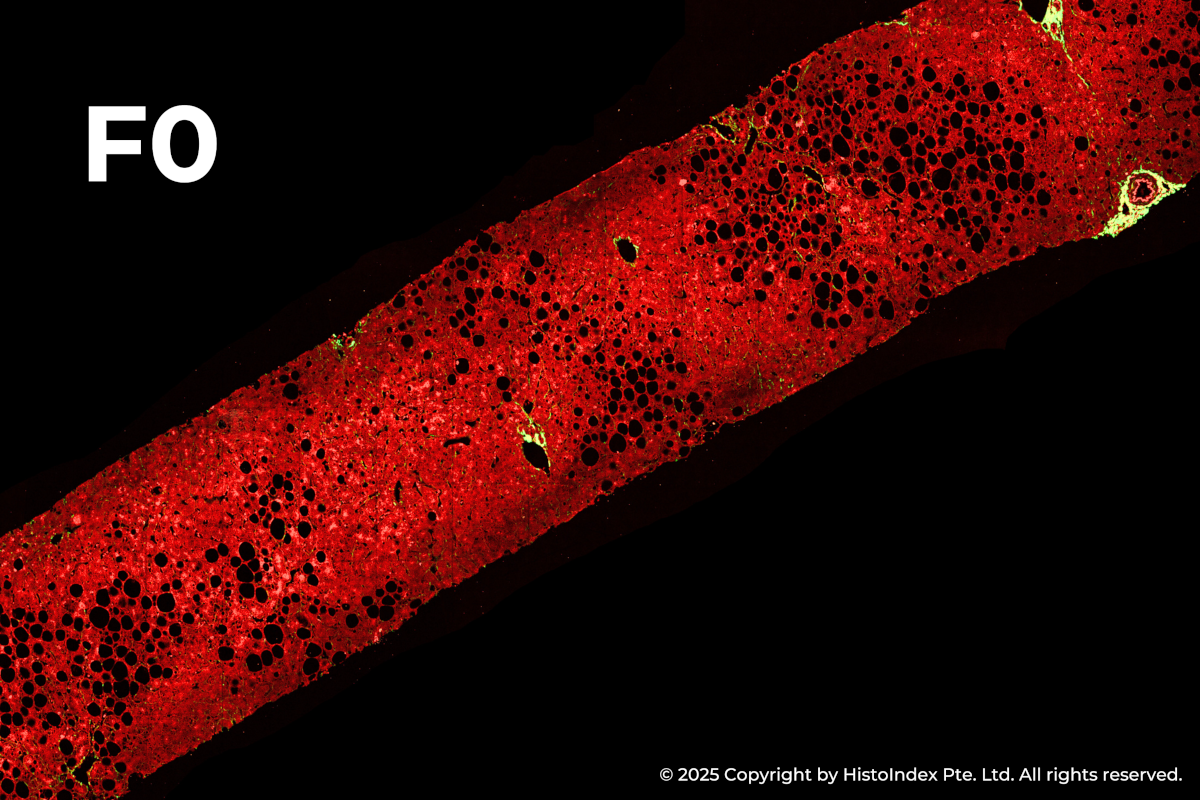
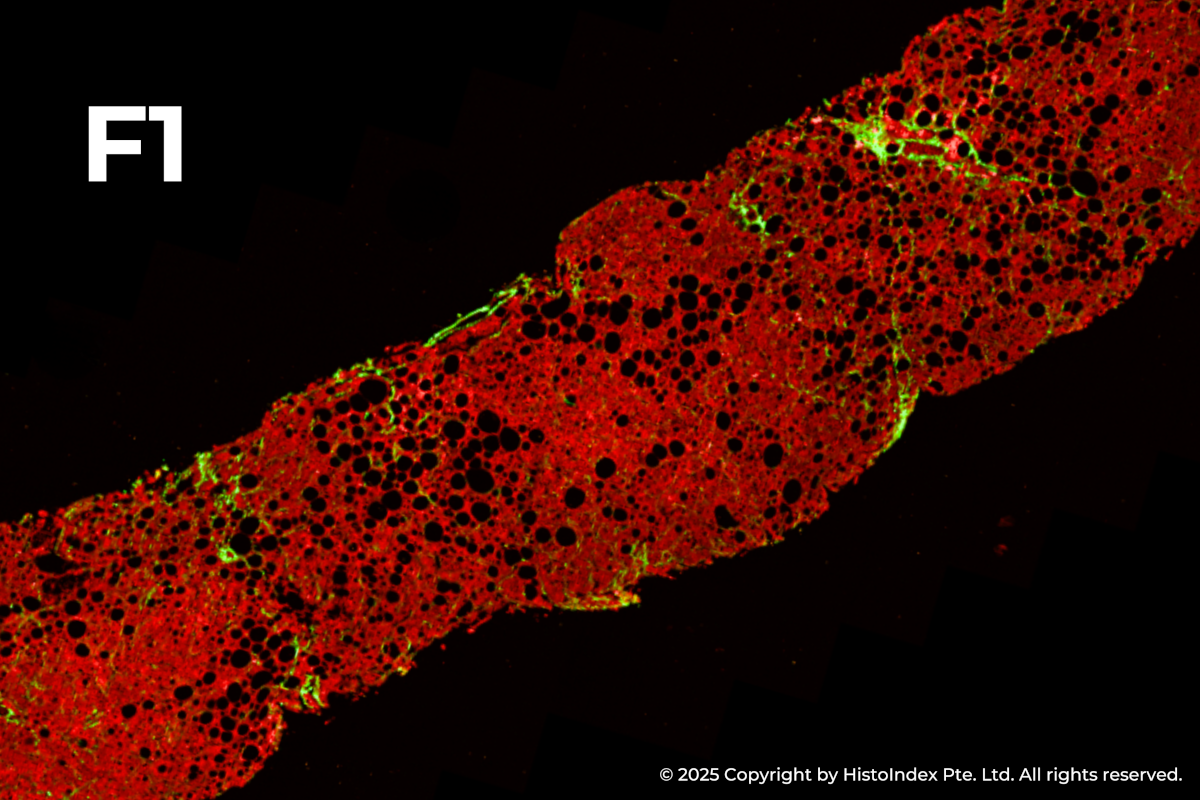
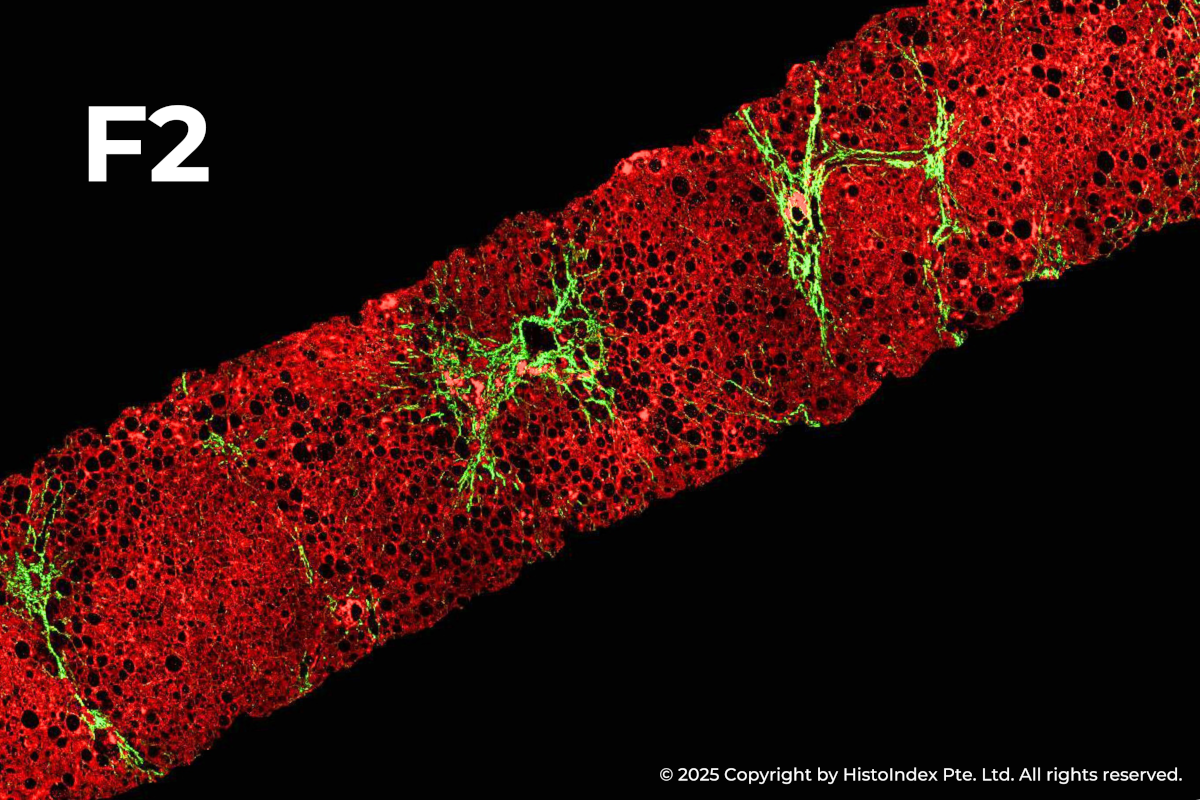
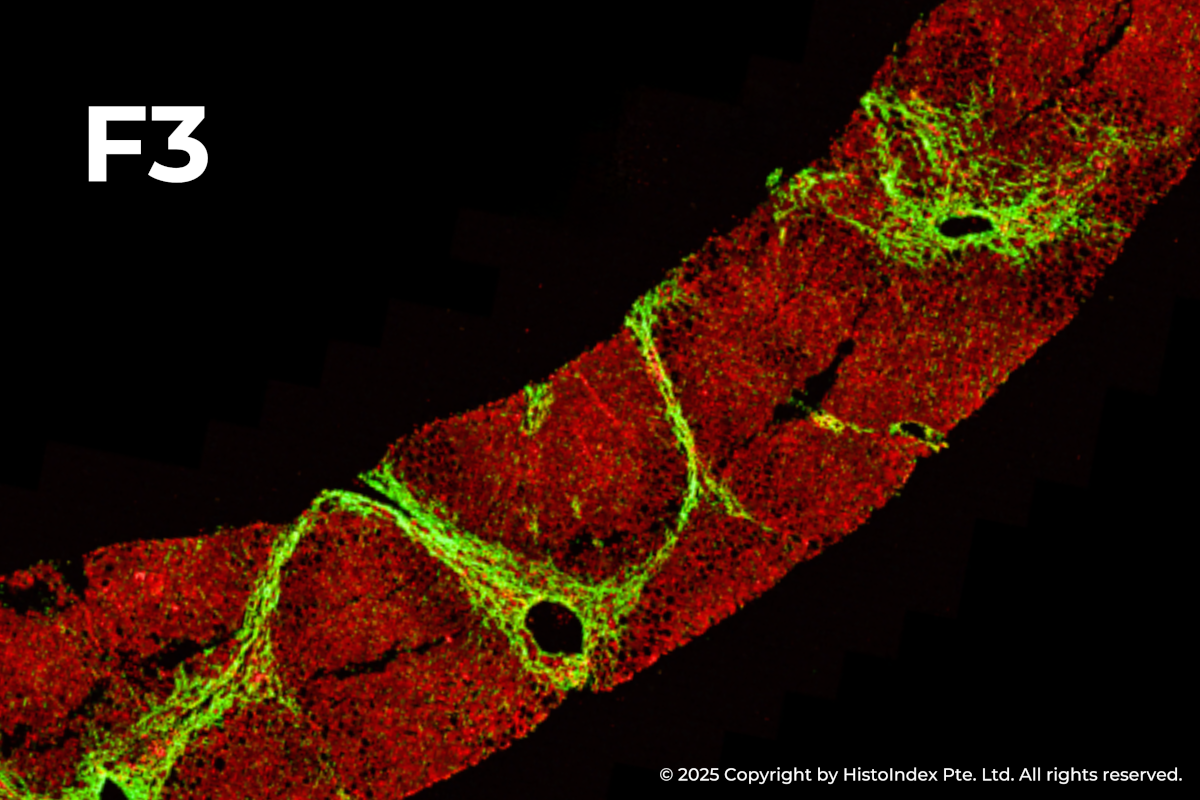
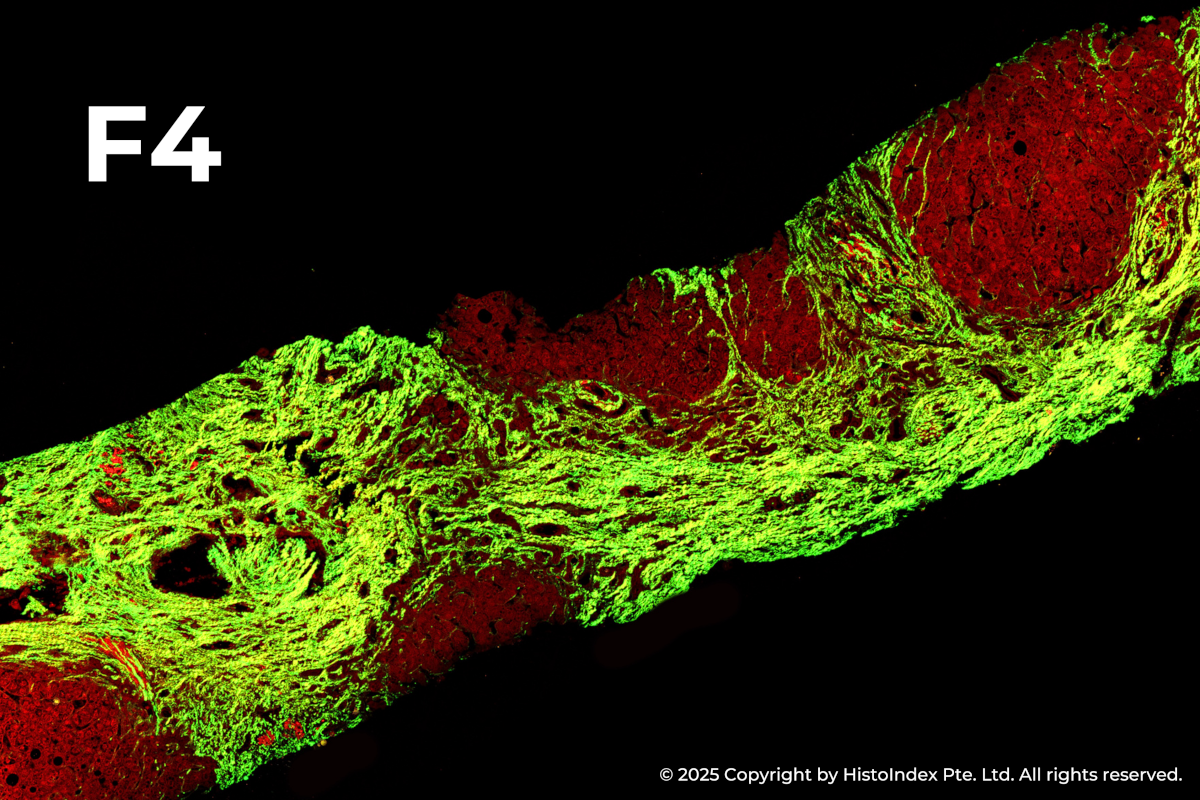
Liver biopsies of fibrosis stages 0 to 4, by NASH-CRN staging system

