AssureMDx For Bladder Cancer Detection
Test Introduction
Test Description
How AssureMDx™ Works
AssureMDx is a proprietary multi-omic test that interrogates the DNA methylation status of OTX1, ONECUT2 and TWIST1 genes in combination with the somatic mutation status of FGFR3, TERT and HRAS genes for the detection of bladder cancer signal in a urine sample.
AssureMDx combines the power of these DNA biomarkers with the addition of clinical risk factors to determine a patient’s risk of having bladder cancer detected upon cystoscopy.
References:
1) van Kessel et al, J Uro 2016, 2) van Kessel et al J Uro 2017, 3) de Jong et al, J Uro 2020
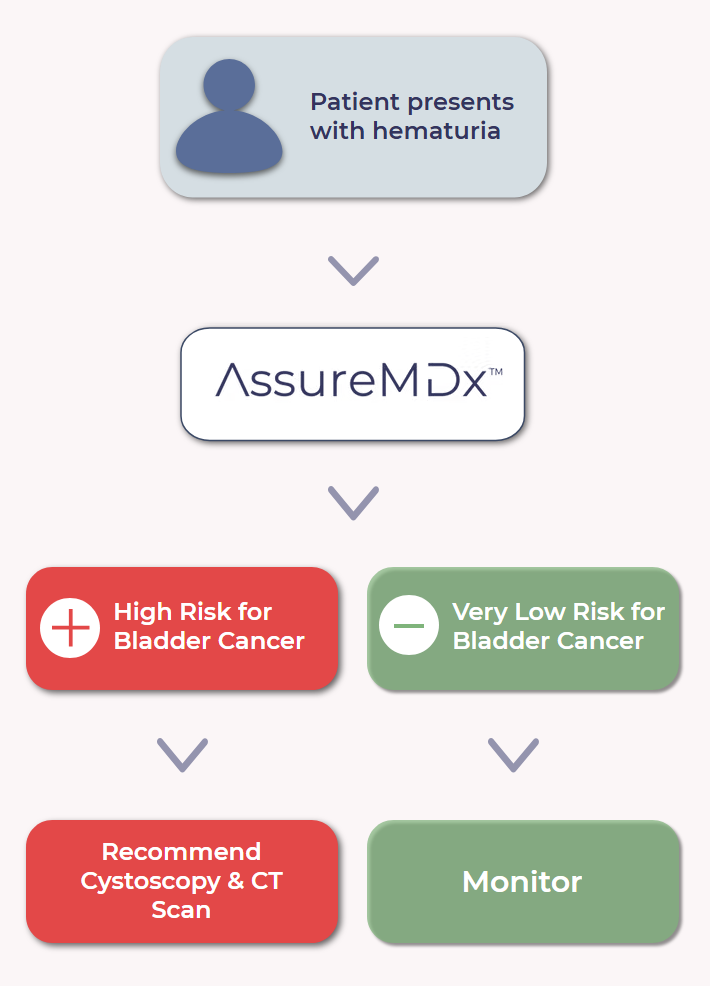
Test Summary:
Clinically Validated Performance
AssureMDx genes & technology have been validated and reported in:- 22 peer-reviewed, published studies
- >6,000 patients
- 0.96 Area Under Curve
- 96% Sensitivity
- 99% Negative Predictive Value
Year | Authors | Journal | Subjects |
|---|---|---|---|
2024 | de Jong et al | European Urology Open | 143 |
2023 | de Jong et al | European Urology Oncology | Review |
2020 | van Kessel et al | Journal of Urology | 838 |
2017 | van Kessel et al | Journal of Urology | 200 |
2017 | Beukers et al | Journal of Urology | 977 |
2016 | van Kessel et al | Journal of Urology | 154 |
2014 | Allory et al | European Urology | 468 |
2013 | Yegin et al | DNA & Cell Biology | 71 |
2013 | Beukers et al | PLOS One | 169 |
2013 | Zuiverloon et al | Journal of Urology | 136 |
2013 | van Kessel et al | Journal of Urology | 70 |
2013 | Kandimalla et al | Clinical Cancer Research | 445 |
2013 | Bangma et al | European Urology | 409 |
2012 | Kamidalla et al | European Urology | 274 |
2011 | Zuiverloon et al | Journal of Urology | 18 |
2011 | Zuiverloon et al | BJU International | 112 |
2010 | Zuiverloon et al | Clinical Cancer Research | 200 |
2010 | Kompier et al | PLOS One | 257 |
2010 | van der Aa et al | Journal of Urology | 448 |
2010 | Renard et al | European Urology | 466 |
2005 | van Oers et al | Clinical Cancer Research | 92 |
2003 | van Rhijn et al | Clinical Cancer Research | 74 |
Test Ordering
AssureMDx is available for purchase directly through the Vesica Health website. This test was developed, and its analytical performance characteristics have been determined by Vesica Health. The test is performed in the Vesica Health Clinical Laboratory located in the ResearchDx Incubator Facility. It has not been cleared or approved by FDA. This assay has been validated pursuant to the CLIA regulations and is used for clinical purposes.
Sample Collection and Shipping Instructions
AssureMDx is available for purchase directly through the Vesica Health website. The test kit contains all required materials for urine collection and shipping the kit to Vesica Health Laboratory for analysis.


